Proof of concept for your mAb in 8 Weeks!
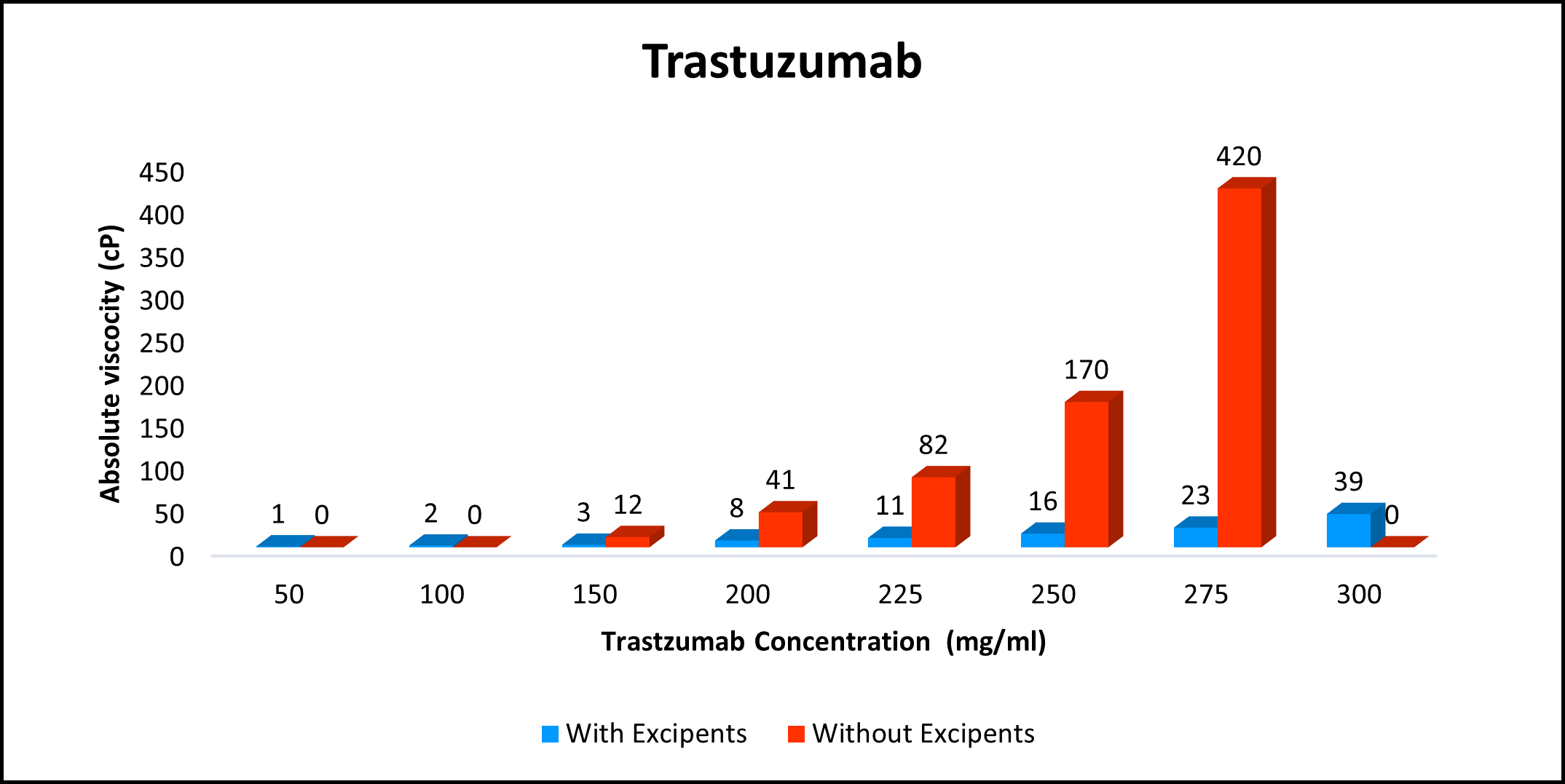
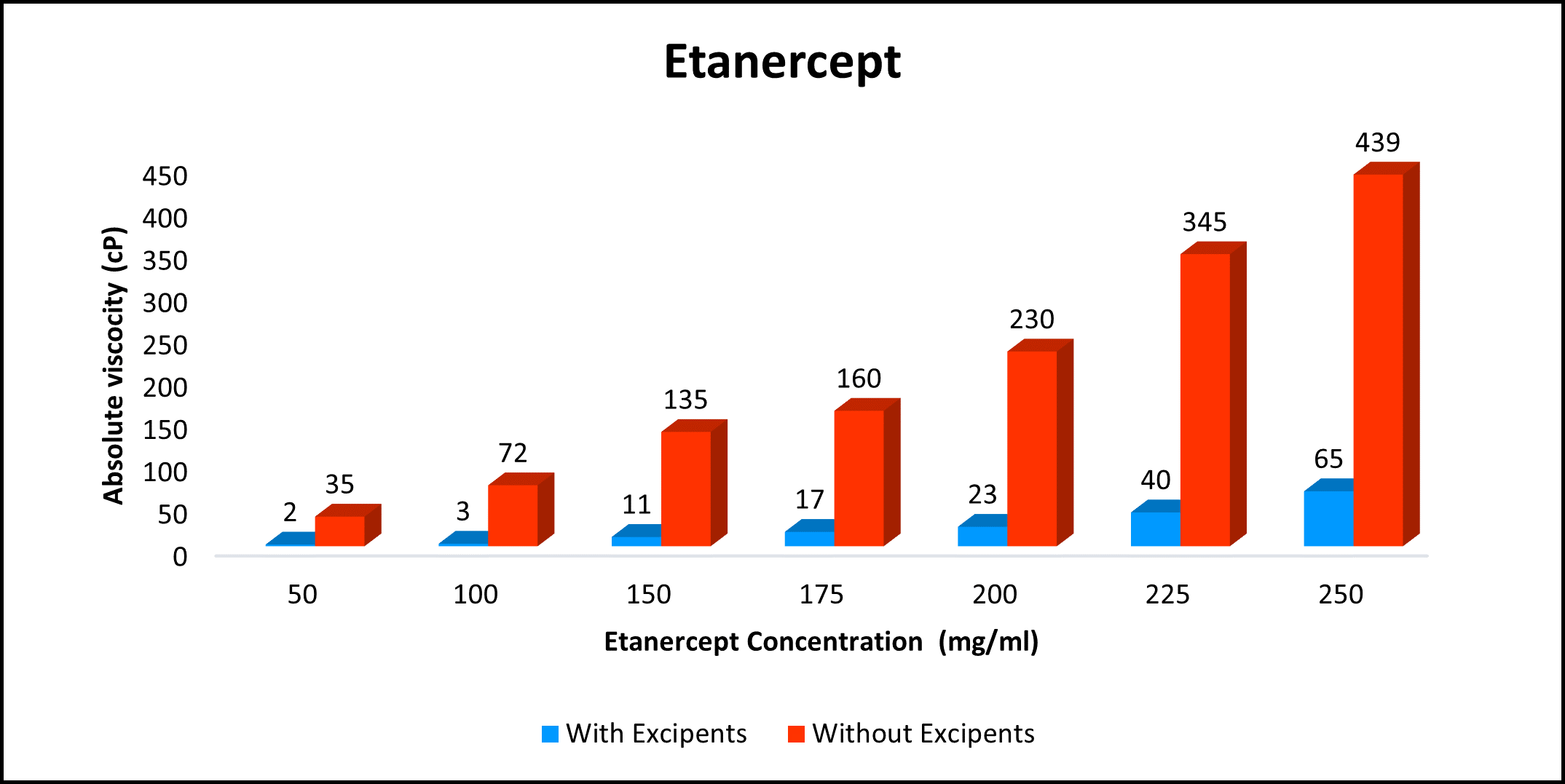
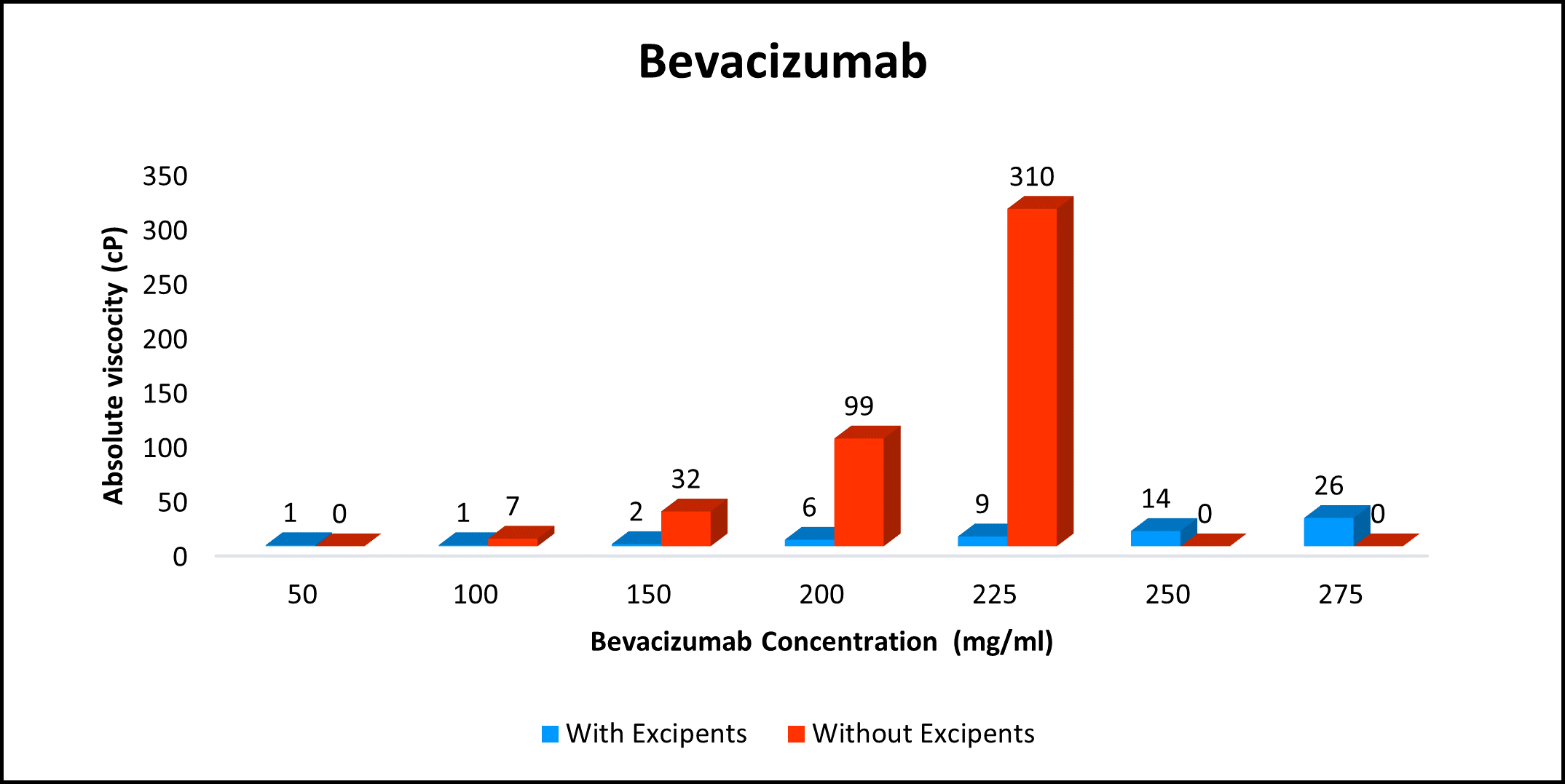
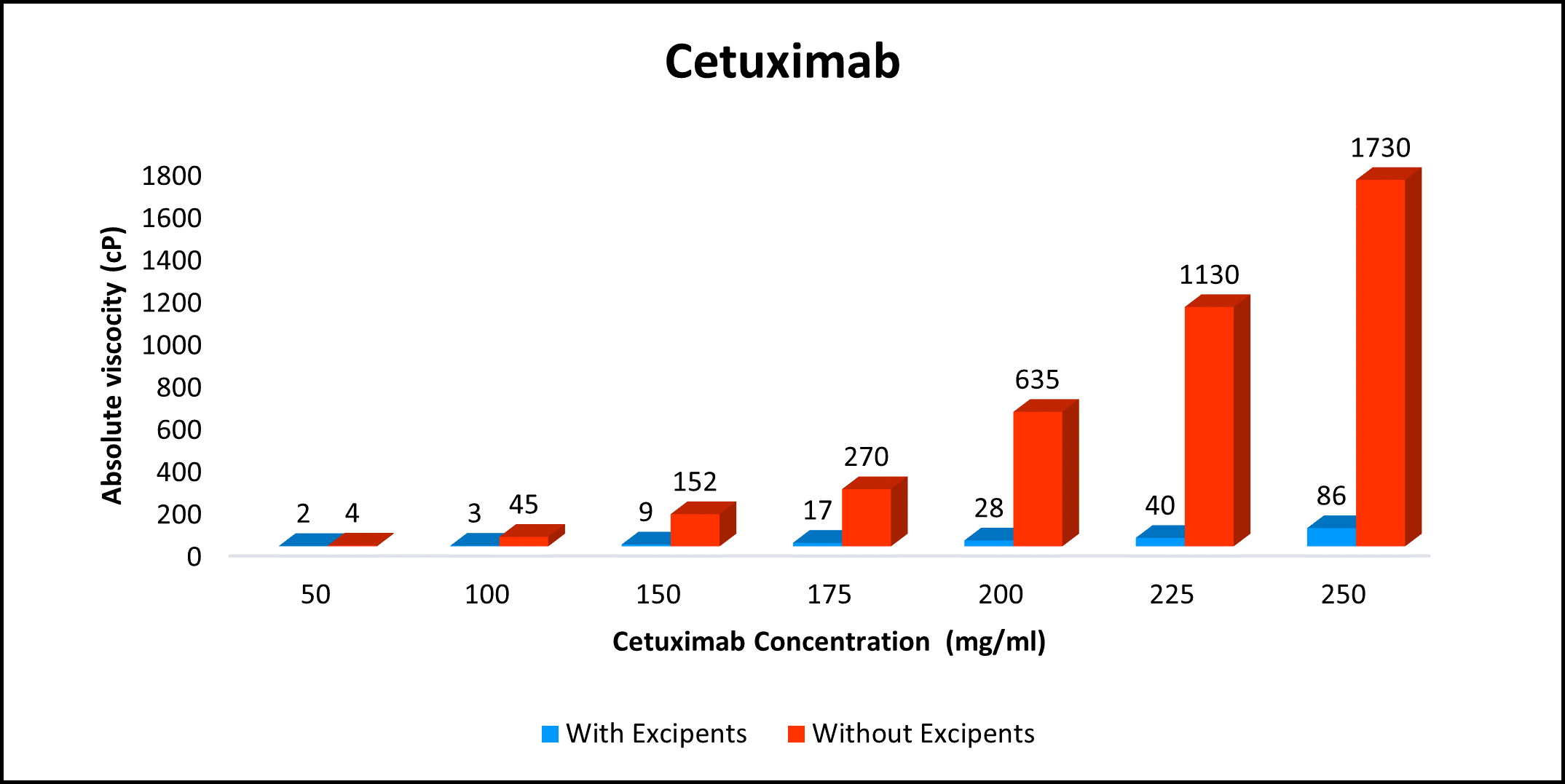
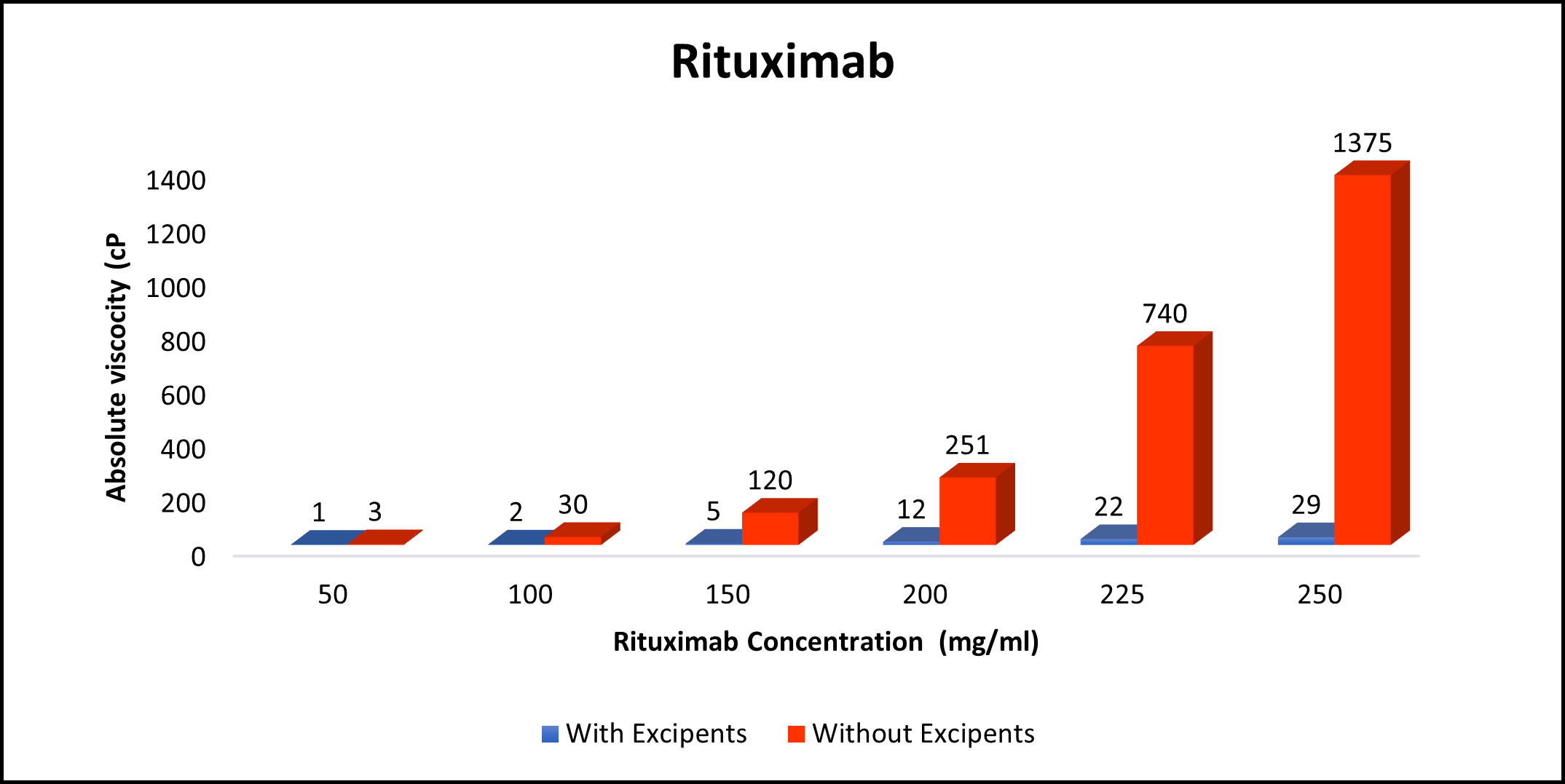
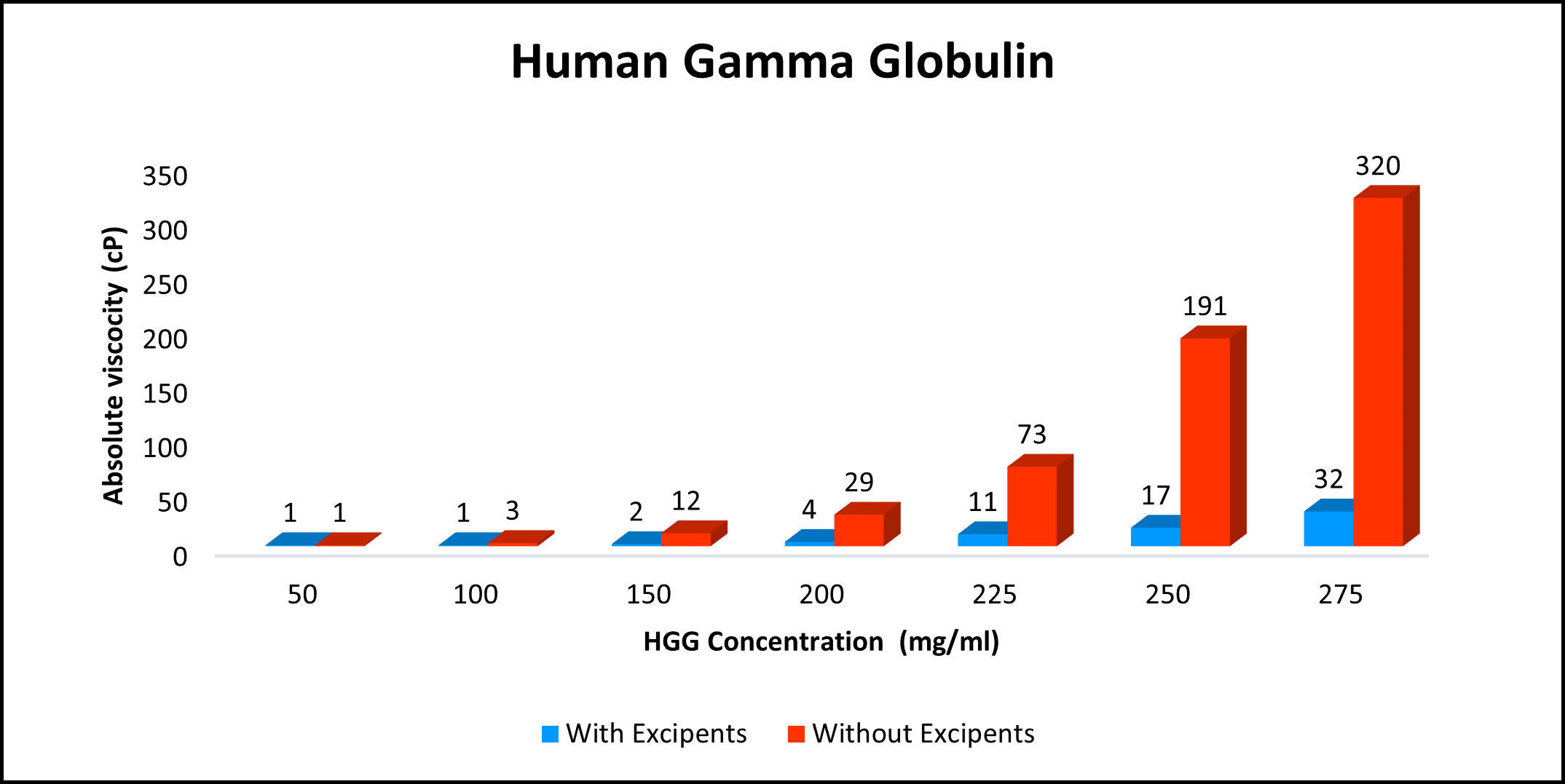
High concentration, low viscosity, and low-volume dosing open entirely new delivery possibilities. HILOPRO® Tech enables biotherapeutics companies to expand their market share and maximize value creation through product life extension.